Marknadsnyheter
Beyfortus approved in the EU for the prevention of RSV lower respiratory tract disease in infants
First and only single-dose RSV preventative option approved for broad newborn and infant population.
European Commission grants first approval worldwide following positive CHMP opinion in September.
AstraZeneca and Sanofi’s Beyfortus (nirsevimab) has been approved in the European Union (EU) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants during their first RSV season.1 Beyfortus is the first and only single-dose RSV passive immunisation for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions.
RSV is a common and highly contagious seasonal virus, infecting nearly all children by the age of two.2,3
The European Commission is the first regulatory body to grant approval to Beyfortus.1 The approval was based on results from the Beyfortus clinical development programme, including the MELODY Phase III, MEDLEY Phase II/III and Phase IIb trials,1,4-11and follows the recommendation by The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency in September 2022.12
In the pivotal MELODY efficacy trial, Beyfortus met its primary endpoint of reducing the incidence of medically attended lower respiratory tract infections (LRTI) caused by RSV by 74.5% (95% CI 49.6, 87.1; p<0.001) vs. placebo through day 151 (a typical RSV season) with a single dose.1,4-9 Beyfortus also demonstrated a comparable safety and tolerability profile to Synagis (palivizumab) in the MEDLEY Phase II/III trial, with occurrence of treatment emergent adverse events (TEAEs) or treatment emergent serious adverse events (TESAEs) similar between groups.1,10-13
Silke Mader, Chairwoman of the Executive Board and Co-founder of the European Foundation for the Care of Newborn Infants (EFCNI), said: “Respiratory syncytial virus represents a health threat among infants, and each year we see the impact it can have on families, healthcare providers and the healthcare system. At EFCNI, we are excited about the opportunity to expand prevention efforts to all infants, as we believe this can help ease the current emotional, physical and financial burdens of RSV.”
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, said: “Beyfortus is the first single-dose preventative option against respiratory syncytial virus to gain approval in Europe and is also the first and only preventative option approved for a broad infant population. Today’s marketing authorisation of Beyfortus marks a significant achievement for the scientific community and addresses a persistent, global unmet need in RSV prevention.”
Thomas Triomphe, Executive Vice President, Vaccines, Sanofi, said: “Today is a landmark day for RSV prevention, as decades of research and development come together in the world’s first approval of a broadly protective option against respiratory syncytial virus disease. Once launched, Beyfortus will offer parents the ability to help protect their babies during their first RSV season.”
RSV is the most common cause of LRTI, including bronchiolitis and pneumonia in infants.14 It is also a leading cause of hospitalisation in all infants.15-18 Globally, in 2019, there were approximately 33 million cases of acute lower respiratory infections leading to more than three million hospitalisations, and it was estimated that there were 26,300 in-hospital deaths of children younger than five years.19 RSV-related direct medical costs, globally – including hospital, outpatient and follow-up care – were estimated at €4.82 billion in 2017.21
Notes
Beyfortus
Beyfortus (nirsevimab), a long-acting antibody designed for all infants for protection against RSV disease from birth through their first RSV season with a single dose, is being developed jointly by AstraZeneca and Sanofi using AstraZeneca’s YTE technology.
Beyfortus has been developed to offer newborns and infants direct RSV protection via an antibody to help prevent LRTI caused by RSV. Monoclonal antibodies do not require the activation of the immune system to help offer timely, rapid and direct protection against disease.20
Beyfortus has been granted marketing authorisation in the European Union for the prevention of RSV LRTI disease in newborns and infants from birth during their first RSV season. The recommended dose of Beyfortus is a single intramuscular injection of 50 mg for infants with body weight <5 kg and a single intramuscular injection of 100 mg for infants with body weight ≥5 kg.12
Beyfortus has also been granted regulatory designations to facilitate expedited development by several major regulatory agencies around the world. These include Breakthrough Therapy Designation by the China Center for Drug Evaluation under the National Medical Products Administration; Breakthrough Therapy Designation from the US Food and Drug Administration; access granted to the European Medicines Agency (EMA PRIority Medicines (PRIME) scheme; and named “a medicine for prioritized development” under the Project for Drug Selection to Promote New Drug Development in Pediatrics by the Japan Agency for Medical Research and Development (AMED). The safety and efficacy of Beyfortus was evaluated under an accelerated assessment procedure by the EMA.
Pivotal clinical trials
The Phase IIb study was a randomised, placebo-controlled trial designed to measure the efficacy of Beyfortus (nirsevimab) against medically attended LRTI through 150 days postdose. Healthy preterm infants of 29–35 weeks’ gestation were randomised (2:1) to receive a single 50mg intramuscular injection of Beyfortus or placebo.1,4,5
The dosing regimen was recommended based on further exploration of the Phase IIb data. The subsequent Phase III study, MELODY applied the recommended dosing regimen.1,3,6
The MELODY Phase III study was a randomised, placebo-controlled trial conducted across 21 countries designed to determine efficacy of Beyfortus against medically attended LRTI due to RSV confirmed by reverse transcriptase polymerase chain reaction testing through 150 days after dosing, versus placebo, in healthy late preterm and term infants (35 weeks gestational age or greater) entering their first RSV season.1-3
MEDLEY was a Phase II/III, randomised, double-blind, Synagis-controlled trial with the primary objective of assessing safety and tolerability for Beyfortus in preterm infants and infants with congenital heart disease (CHD) and/or chronic lung disease of prematurity (CLD) eligible to receive Synagis.1,8,9 Between July 2019 and May 2021 approximately 918 infants entering their first RSV season were randomised to receive a single 50mg (in infants weighing <5kg) or 100mg (in infants weighing ≥5kg) intramuscular injection of Beyfortus or Synagis. Safety was assessed by monitoring the occurrence of TEAEs and TESAEs through 360 days post-dose.1,8,9 Serum levels of Beyfortus following dosing (on day 151) in this trial were comparable with those observed in the MELODY Phase III trial, indicating similar protection in this population to that in the healthy term and late preterm infants is likely. Data was published in the New England Journal of Medicine (NEJM) in March 2022.
The results of MELODY, MEDLEY Phase II/III and the Phase IIb trials demonstrate that Beyfortus helps protect infants during their first RSV season against RSV disease with a single dose.1-9 This all-infant population includes preterm, healthy late preterm and term infants, as well as infants with specific conditions.
These trials form the basis of regulatory submissions which began in 2022.
Results from the Phase IIb trial
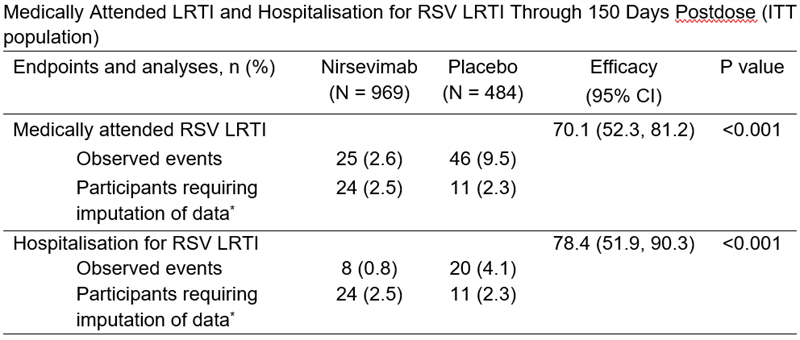
The primary endpoint of the Phase IIb study was met, reducing the incidence of medically attended LRTI, caused by RSV by 70.1% (95% CI: 52.3, 81.2) compared to placebo. Between November 2016 and December 2017, 1,453 infants were randomised (Beyfortus, n=969; placebo, n=484) at the RSV season start. Research was conducted by AstraZeneca in both hemispheres, at 164 sites in 23 countries.1,4,5 Data was published in NEJM in July 2020.

Data were imputed for participants who had no events and were not followed through 150 days postdose. Analyses were conducted using Poisson regression with robust variance. CI, confidence interval; ITT, intent-to-treat; LRTI, lower respiratory tract infection; RRR, relative risk reduction; RSV, respiratory syncytial virus.
Results from the MELODY Phase III trial
The primary endpoint of the MELODY Phase III trial was met, reducing the incidence of medically attended LRTI, such as bronchiolitis or pneumonia, caused by RSV by 74.5% (95% CI 49.6, 87.1; P<0.001) compared to placebo. Infants were randomised (2:1) to receive a single 50mg (in infants weighing <5kg) or 100mg (in infants weighing ≥5kg) intramuscular injection of Beyfortus or placebo. Between July 2019 and March 2020, 1,490 infants were randomised to either Beyfortus or placebo at the RSV season start.1-3 Data was published in NEJM in March 2022.

Data were imputed for participants who had no events and were not followed through 150 days postdose. Analyses were conducted using Poisson regression with robust variance. CI, confidence interval; ITT, intent-to-treat; LRTI, lower respiratory tract infection; RRR, relative risk reduction; RSV, respiratory syncytial virus.
Results from the pre-specified pooled analysis of the Phase IIb and MELODY trials
A prespecified pooled analysis of the MELODY Phase III trial and the recommended dose from the Phase IIb trial, in which an efficacy (relative risk reduction versus placebo) of 79.5% (95% CI 65.9, 87.7; P<0.0001) was seen against medically attended LRTI, such as bronchiolitis or pneumonia, caused by RSV in infants born at term or preterm entering their first RSV season.1,8 The pooled analysis studied healthy preterm and term infants who received the recommended dose of Beyfortus based on weight compared to placebo through Day 151 and showed an efficacy of 77.3% (95% CI 50.3, 89.7; P<0.001) against RSV LRTI hospitalisations.1,4,8
Medically Attended LRTI and Hospitalisation for RSV LRTI Through 150 Days Postdose (ITT population)

Data were imputed for participants who had no events and were not followed through 150 days postdose. Analyses were conducted using Poisson regression with robust variance. CI, confidence interval; ITT, intent-to-treat; LRTI, lower respiratory tract infection; RRR, relative risk reduction; RSV, respiratory syncytial virus.
Sanofi Alliance
In March 2017, AstraZeneca and Sanofi announced an agreement to develop and commercialise nirsevimab. Under the terms of the agreement, AstraZeneca leads all development and manufacturing activities, and Sanofi leads commercialisation activities and records revenue. Under the terms of the global agreement, Sanofi made an upfront payment of €120m, has paid a development milestone of €30m and will pay up to a further €465m upon achievement of certain development and sales-related milestones. The two companies share costs and profits. Revenue from the agreement is reported as Collaboration Revenue in the Company’s financial statements.
Sobi agreement
Related, in November 2018, AstraZeneca agreed to sell US commercial rights for Synagis (palivizumab) to Swedish Orphan Biovitrum AB (publ) (Sobi) in addition to the right to participate in payments that may be received by AstraZeneca from the US profits or losses for nirsevimab. Under the agreement AstraZeneca received upfront consideration and also received non-contingent payments for nirsevimab during 2019-2021. AstraZeneca is also entitled to receive certain milestone payments for nirsevimab, including a $175m milestone following the date on which the Biologics License Application (BLA) for nirsevimab is accepted for filing by the FDA and a $90m milestone payment following the date on which BLA approval in the US occurs. AstraZeneca will continue to manufacture and supply nirsevimab globally and is entitled to an additional royalty from Sobi if profits from nirsevimab in the US exceed a pre-specified level.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
- European Commission. https://ec.europa.eu/transparency/documentsregister/detail?ref=C(2022)7992&lang=enAccessed November 2022
- Glezen WP et al. Am J Dis Child. 1986;140(6):543-5463.
- Collins et al. Journal of Virology. 2008:2040–2055.
- Hammitt LL, MD et al. Nirsevimab for Prevention of RSV in Healthy Late -Preterm and Term Infants. N Engl J Med. 2022;386 (9): 837-846. doi: 10.1056/NEJMoa2110275.
- Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Late Preterm and Term Infants (MELODY). https://clinicaltrials.gov/ct2/show/NCT03979313. Accessed September 2022.
- Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Preterm Infants. (MEDI8897 Ph2b). https://www.clinicaltrials.gov/ct2/show/NCT02878330 . Accessed September 2022.
- Griffin P, MD et al. (2020). Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. NEJM 2020; 383: 415-425. DOI: 10.1056/NEJMoa1913556.
- Simões, E, et al. Pooled efficacy of nirsevimab against RSV lower respiratory tract infection in preterm and term infants. ESPID 2022 Congress; 2022 May 9-13. Hybrid Congress.
- Wilkins, D, et al. Nirsevimab for the prevention of respiratory syncytial virus infection: neutralizing antibody levels following a single dose. ESPID 2022 Congress; 2022 May 9-13. Hybrid Congress.
- Domachowske J, MD et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N Engl J Med. 2022; 386 (9).
- Clinicaltrials.gov. A Study to Evaluate the Safety of MEDI8897 for the Prevention of Medically Attended Respiratory Syncytial Virus (RSV) Lower Respiratory Track Infection (LRTI) in High-risk Children. https://clinicaltrials.gov/ct2/show/NCT03959488 (MEDLEY). Accessed September 2022.
- European Medicines Agency. Beyfortus Summary of Committee for Medicinal Products for Human Use Opinion Available at: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/beyfortus. Accessed September 2022.
- Synagis – Summary of Product Characteristics (SmPC) – (eMC) [Internet]. Available from: https://www.medicines.org.uk/emc/product/6963/smpc Accessed September 2022.
- R K. Respiratory Syncytial Virus Vaccines. Plotkin SA, Orenstein WA, Offitt PA, Edwards KM, eds Plotkin’s Vaccines 7th ed Philadelphia. 2018;7th ed. Philadelphia:943-9.
- Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. The Pediatric infectious disease journal. 2002;21(7):629-32.
- McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. Journal of Perinatology: official journal of the California Perinatal Association. 2016;36(11):990-6.
- Rha B, et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015-2016. Pediatrics. 2020;146:e20193611.
- Arriola CS, et al. Estimated Burden of Community-Onset Respiratory Syncytial Virus-Associated Hospitalizations Among Children Aged <2 Years in the United States, 2014-15. J Pediatric Infect Dis Soc. 2020;9:587-595
- Li Y, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:92047–64.
- Centers for Disease Control and Prevention. Vaccines & Immunizations. August 18, 2017. https://www.cdc.gov/vaccines/vac-gen/immunity-types.htm. Accessed September 2022.
- Zhang S, et al. Cost of Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection Management in Young Children at the Regional and Global Level: A Systematic Review and Meta-Analysis. J Infect Dis. 2020;222(Suppl 7):S680-687.
Marknadsnyheter
Greater Thans årsredovisning för 2023 har publicerats


Årsredovisningen finns tillgänglig på https://greaterthan.eu/resources/press-release-category/regulatory/
Presskontakt Greater Than
PR@greaterthan.eu
+46 855 593 200
www.greaterthan.eu
Om Greater Than
Greater Than är ett dataanalysföretag som är specialiserat på att förstå bilförares påverkan på vägarna. Genom kraften av artificiell intelligens (AI) omvandlar Greater Than GPS-data till insikter som förutspår förarens sannolikhet att krocka och dess klimatpåverkan.
Försäkringsbolag, bilflottor, transportföretag och andra ägare av GPS-data använder Greater Than:s dataanalys för att optimera riskhantering av förare, försäkringslönsamhet samt hantera hållbarhets- och ESG-rapportering.
Greater Than har utsetts på de globala InsurTech100 och AIFinTech100 listorna. Greater Than (GREAT) är noterat på Nasdaq First North Growth Market. FNCA Sweden AB är bolagets Certified Adviser. Läs mer på www.greaterthan.eu.
Taggar:
Marknadsnyheter
Bokslutskommentar från Setra för det första kvartalet 2024
Träindustriföretaget Setra redovisar för första kvartalet 2024 ett rörelseresultat på -95 (-64) Mkr. Nettoomsättningen uppgick till 1 158 (1 414) Mkr.
– Sågverksindustrin befinner sig fortsatt i en besvärlig situation med höga timmerpriser och en svag byggkonjunktur. Pågående investeringar i kombination med en kall och utdragen vinter har påverkat produktionsvolymen negativt. Tack vare en väl balanserad marknadsmix har Setra inga svårigheter att hitta avsättning för det vi producerar och vi räknar med ökande försäljningspriser under kommande kvartal, säger Marcus Westdahl, Setras VD.
Installationen av en ny klentimmerlinje i Skinnskatteberg är inne i sitt slutskede och den första stocken sågades i slutet av januari. Parallellt har nästa steg i Setras anläggningsstrategi inletts, där anläggningen i Malå kommer att få ett nytt timmerintag, en ny såglinje, ett uppdaterat justerverk och en ny torkanläggning. Beslut om investeringen i Malå togs i oktober 2022.
Setra levererar trästommarna till den första etappen av Greenhouse Sthlm. Det är Electroluxkoncernens nya klimatssmarta kvarter som rymmer både huvudkontor samt bostäder, restaurang och butiker.
I mitten av mars informerade Setra om invigningen av en ny bearbetningsmaskin för korslimmat trä. Därmed fördubblas kapaciteten vid KL-träfabriken i Långshyttan. I takt med samhällets gröna omställning växer intresset för att bygga i trä. Setra vill driva utvecklingen framåt i branschen genom att satsa på standardiserade lösningar som förenklar för kunderna.
Kassaflödet från den löpande verksamheten för det första kvartalet uppgick till -226 (-82) Mkr.
| Nyckeltal* | jan-mars (3 mån) | ||||
| 2024 | 2023 | ||||
| Nettoomsättning, Mkr | 1 158 | 1 414 | |||
| Rörelseresultat, Mkr | -95 | -64 | |||
| Resultat efter skatt, Mkr | -81 | -49 | |||
| Rörelsemarginal, % | -8,2 | -4,5 | |||
| Avkastning på operativt kapital, %, RTM | -11 | 30 | |||
| Kassaflöde från löpande verksamhet, Mkr | -226 | -82 | |||
*Setra redovisar ingen fullständig bokslutskommuniké.
För ytterligare information, kontakta gärna:
Marcus Westdahl, VD och koncernchef, telefon 08-705 03 75, mobil 073-098 14 00
Johanna Gydingsgård, CFO, telefon 08-705 03 03, mobil 072-453 85 41
På Setra vill vi vara grönsamma. När en verksamhet är lönsam för alla, kallar vi det för Grönsamhet. Setra är ett av Sveriges största träindustriföretag. Vi förädlar råvara från ansvarsfullt brukade skogar till klimatvänliga produkter såsom limträ, hyvlat, komponenter, KL-trä och sågade trävaror. Vi säljer även bioprodukter som bark, flis och spån till kunder inom pappers- och massaindustrin samt till energiframställning. Våra kunder finns globalt. Läs gärna mer på setragroup.com
Marknadsnyheter
Ny rapport om svenskt arbetsliv! Aldrig har svenskt arbetsliv varit så attraktivt och jämställt som 2023!
Modity Energy Trading AB, Fujitsu Sweden AB och Sveriges Riksbank har bäst arbetsvillkor i landet. Det visar ny statistik från Nyckeltalsinstitutet som kartlagt de mest attraktiva, jämställda och hälsofrämjande arbetsgivarna i Sverige. Resultaten baseras på index som är genererade från drygt 800 000 medarbetares rådande arbetsvillkor. Ta del av 2023 års ”Topp tre” arbetsgivare i landet – per kategori.
Nya data från Nyckeltalsinstitutet visar att en allt högre andel medarbetare i svenskt arbetsliv har en trygg anställning, många arbetsgivare investerar mer i kompetensutveckling, samt att rekryterings och introduktionsprocesserna tycks ha blivit bättre. Efter ett par års uppgång i både sjukfrånvaro och avgångstal har båda nu bromsat in. Även inom jämställdhet har många områden inom svenskt arbetsliv blivit något mer jämställda. Grundstrukturerna med fördelningen kvinnor och män börjar långsamt jämnas ut men det är fortsatt stora skillnader i förändringstakten mellan mansdominerade och kvinnodominerade verksamheter.
Det finns fortfarande några utmanande iakttagelser såsom skillnaden i långtidssjukfrånvaro mellan män och kvinnor, den offentliga sektorns tendens att ha många anställda per chef och utmaningen med att förändra homogena könsstrukturer.
Alla styrkor och utmaningar i svenskt arbetsliv har en prislapp, det finns stora värden i att lyckas kontra kostnaderna för insatserna. Mer om dessa ekonomiska resonemang finns i vår årsrapport. Ta del av hela kartläggningen tillsammans med ännu mer intressanta resultat och analyser i Nyckeltalsinstitutets årsrapport: www.nyckeltal.se/kunskapsbank/
– Medarbetarna är organisationens viktigaste resurs, oavsett vilket läge organisationen befinner sig i. I årets databas ser vi att ju fler nyanställda som är kvar efter sitt första år, desto lägre avgångar hos alla medarbetare. Det ger oss ett riktigt bra kvitto på att vi som arbetsgivare uppfyller de förväntningar som råder, att vi är så bra som vi säger att vi är! Att kontinuerligt mäta och ha förståelse för hur de egna medarbetarnas arbetsvillkor ser ut, är en nyckel för att kunna styra sin verksamhet framåt tillsammans med sina medarbetare, säger Tina Ekström, vd på Nyckeltalsinstitutet.
Utnämningarna baseras på insamlad HR statistik från 2023 med information om drygt 800 000 medarbetares faktiska arbetsvillkor, från cirka 400 företag och organisationer.
HÄR ÄR 2023 ÅRS ”TOPP TRE” ARBETSGIVARE I LANDET – PER KATEGORI:
Topplistan i Attraktiv Arbetsgivarindex® 2023:
- Modity Energy Trading AB
- Uniper Sverige
- Hewlett Packard Enterprise
Topplistan i Jämställdhetsindex JÄMIX® 2023:
- Fujitsu Sweden AB
- Exploateringskontoret Stockholms Stad
- Canon Svenska AB
Topplista i Hälsoindex™ 2023:
- Sveriges Riksbank
- Riksrevisionen
- Bankgirot
Kategorin Attraktiv arbetsgivarindex baseras på faktorer som bland annat trygga anställningsvillkor, personalomsättning, chefstäthet, kompetensutveckling och sjukfrånvaro. Jämställdhetsindex baseras på faktorer om lika arbetsvillkor för män och kvinnor som t.ex. jämställda lönestrukturer, jämställd ledningsgrupp, lika karriärmöjligheter, föräldraskap och anställningsformer. Hälsoindex baseras bland annat på faktorerna frisktal, rehabresultat, sjukfrånvaro, friskvård och arbetsmiljöarbete.
För en intervju och förklaring av rapporten, kontakta gärna:
Anders Johrén, analytiker och sakkunnig på Nyckeltalsinstitutet. 070 – 626 28 26, anders@nyckeltal.se
Tina Ekström, VD, Nyckeltalsinstitutet, 070-453 80 81, tina@nyckeltal.se
Om Nyckeltalsinstitutet
Sedan 1996 har Nyckeltalsinstitutet genom vetenskapliga mätmetoder samlat in, kartlagt och rapporterat HR ekonomisk data till viktiga beslutsunderlag som skapar förutsättningar till ett arbetsliv med engagerade medarbetare och framgångsrika arbetsgivare. Nyckeltalsinstitutet har idag den största databasen med rådande arbetsvillkor från olika branscher i svenskt arbetsliv. I en värld av tyckande levererar Nyckeltalsinstitutet fakta som skapar framgångsrika arbetsgivare.
Mer information om Nyckeltalsinstitutets verksamhet finns på www.nyckeltal.se
Taggar:
-
Analys från DailyFX8 år ago
EUR/USD Flirts with Monthly Close Under 30 Year Trendline
-

 Marknadsnyheter1 år ago
Marknadsnyheter1 år agoUpptäck de bästa verktygen för att analysera Bitcoin!
-
Marknadsnyheter4 år ago
BrainCool AB (publ): erhåller bidrag (grant) om 0,9 MSEK från Vinnova för bolagets projekt inom behandling av covid-19 patienter med hög feber
-
Analys från DailyFX11 år ago
Japanese Yen Breakout or Fakeout? ZAR/JPY May Provide the Answer
-
Analys från DailyFX11 år ago
Price & Time: Key Levels to Watch in the Aftermath of NFP
-
Analys från DailyFX7 år ago
Gold Prices Falter at Resistance: Is the Bullish Run Finished?
-

 Nyheter5 år ago
Nyheter5 år agoTeknisk analys med Martin Hallström och Nils Brobacke
-
Marknadsnyheter6 år ago
Tudorza reduces exacerbations and demonstrates cardiovascular safety in COPD patients

