Marknadsnyheter
Enhertu approved in the US as the first HER2-directed therapy for patients with HER2-low metastatic breast cancer
Based on DESTINY-Breast04 results which showed AstraZeneca and Daiichi Sankyo’s Enhertu reduced risk of disease progression or death by 50% and increased overall survival by more than six months versus chemotherapy.
AstraZeneca and Daiichi Sankyo’s Enhertu (trastuzumab deruxtecan) has been approved in the US for the treatment of adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy.
Enhertu is a specifically engineered HER2-directed antibody drug conjugate (ADC) being jointly developed and commercialised by AstraZeneca and Daiichi Sankyo.
The approval by the Food and Drug Administration (FDA) was based on the results from the DESTINY-Breast04 Phase III trial. In the trial, Enhertu reduced the risk of disease progression or death by 50% versus physician’s choice of chemotherapy in patients with HER2-low metastatic breast cancer with hormone receptor (HR)-positive disease or HR-negative disease (median progression-free survival [PFS] 9.9 versus 5.1 months; hazard ratio [HR] 0.50; 95% confidence interval [CI] 0.40-0.63; p<0.0001). A median overall survival (OS) of 23.4 months was seen in patients treated with Enhertu versus 16.8 months in those treated with chemotherapy, a 36% reduction in the risk of death (HR 0.64; 95% CI 0.49-0.84; p=0.001).
Shanu Modi, MD, Medical Oncologist, Memorial Sloan Kettering Cancer Center, US, said: “Approximately half of all patients with breast cancer have tumours that are HER2-low, which have previously been classified as HER2-negative and have not had effective treatment options with HER2-targeted medicines. Based on the promising results of the DESTINY-Breast04 trial, clinicians are starting to differentiate levels of HER2 expression and redefine how metastatic breast cancer is classified with a distinct HER2-low patient population that may be eligible for trastuzumab deruxtecan.”
Dave Fredrickson, Executive Vice President, Oncology Business Unit, AstraZeneca, said: “The rapid approval of Enhertu in HER2-low metastatic breast cancer by the FDA underscores the urgency to bring this transformational medicine to patients as quickly as possible. Patients with HER2-low tumours, who are identified through existing HER2 testing methods, will now have the opportunity to be treated based upon their HER2 status.”
Ken Keller, Global Head of Oncology Business and President and CEO, Daiichi Sankyo, Inc, said: “Today’s FDA approval marks a monumental moment in breast cancer treatment as Enhertu is the first-ever HER2-directed medicine to be approved for the treatment of patients with HER2-low metastatic breast cancer. With the ground-breaking survival benefit seen in the DESTINY-Breast04 trial, this milestone confirms the importance of targeting lower levels of HER2 expression in the treatment of metastatic breast cancer and we are thrilled that we can now offer Enhertu to even more patients.”
The approval was granted under the FDA’s Real-Time Oncology Review programme after securing Priority Review and Breakthrough Therapy Designation of Enhertu in the US in this setting. The expanded approval for Enhertu in the US, following its previous approval in 2nd-line HER2-positive metastatic breast cancer, enables its use across a wide spectrum of HER2-expressing breast cancer, including patients with HER2-low disease.
The DESTINY-Breast04 Phase III trial results were presented at the presidential plenary session of the 2022 American Society of Clinical Oncology Annual meeting and simultaneously published in The New England Journal of Medicine (NEJM).1
The safety profile of Enhertu was consistent with previous clinical trials with no new safety concerns identified. Interstitial lung disease (ILD) or pneumonitis rates were consistent with those observed in late-line HER2-positive breast cancer trials of Enhertu. Overall, 12% of patients had confirmed ILD or pneumonitis related to treatment as determined by an independent adjudication committee. The majority of ILD events were Grade 1 or 2 (10%), with five Grade 3 (1.3%) and no Grade 4 events reported. There were three (0.8%) ILD-related deaths (Grade 5).
In June 2022, fam-trastuzumab deruxtecan-nxki (Enhertu) was added to the NCCN Clinical Practical Guidelines in Oncology (NCCN Guidelines®) as the Category 1 preferred regimen for patients with tumours that are HER2 IHC 1+ or 2+ and ISH- who have received at least one prior line of chemotherapy for metastatic disease and, if tumour is HR-positive, are refractory to endocrine therapy, based on the data from DESTINY-Breast04.2
The US regulatory submission for DESTINY-Breast04 was reviewed under Project Orbis, which provides a framework for concurrent submission and review of oncology medicines among participating international partners. As part of Project Orbis, Enhertu is also under regulatory review for the same indication by the Australian Therapeutic Goods Administration, the Brazilian Health Regulatory Agency (ANVISA), Health Canada and Switzerland’s Swissmedic.
Regulatory applications for Enhertu are also currently under review in Europe, Japan and several other countries based on the DESTINY-Breast04 results.
Notes
Financial considerations
Following this approval for Enhertu in the US, an amount of $200m is due from AstraZeneca to Daiichi Sankyo as a milestone payment for the HER2-low breast cancer post chemotherapy indication. The milestone will be capitalised as an addition to the upfront payment made by AstraZeneca to Daiichi Sankyo in 2019 and subsequent capitalised milestones, and will be amortised through the profit and loss statement.
Sales of Enhertu in the US are recognised by Daiichi Sankyo. AstraZeneca reports its share of gross profit margin from Enhertu sales in the US as collaboration revenue in the Company’s financial statements.
Further details on the financial arrangements were set out in the March 2019 announcement of the collaboration.
Breast cancer and HER2 expression
Breast cancer is the most common cancer and is one of the leading causes of cancer-related deaths worldwide and in the US.3,4 More than two million patients with breast cancer were diagnosed in 2020 resulting in nearly 685,000 deaths globally.3 In the US, more than 290,000 patients are expected to be diagnosed in 2022, resulting in more than 43,000 deaths.5
HER2 is a tyrosine kinase receptor growth-promoting protein expressed on the surface of many types of tumours including breast, gastric, lung and colorectal cancers, and is one of many biomarkers expressed in breast cancer tumours.6
HER2 expression is currently determined by an immunohistochemistry (IHC) test which estimates the amount of HER2 protein on a cancer cell, and/or an in-situ hybridisation (ISH) test, which counts the copies of the HER2 gene in cancer cells.6,7 HER2 tests provide IHC and ISH scores across the full HER2 spectrum and are routinely used to determine appropriate treatment options for patients with metastatic breast cancer.
HER2-positive cancers are currently defined as HER2 expression measured as IHC 3+ or IHC 2+/ISH+, and HER2-negative cancers are defined as HER2 expression measured as IHC 0, IHC 1+ or IHC 2+/ISH-.6 However, approximately half of all breast cancers are HER2-low, defined as an HER2 score of IHC 1+ or IHC 2+/ISH-.8-10 HER2-low occurs in both HR-positive and HR-negative disease.11
Previously, patients with HR-positive metastatic breast cancer and HER2-low disease had limited effective treatment options following progression on endocrine (hormone) therapy.9,12 Additionally, few targeted options were available for those with HR-negative disease.13 Now with the approval of Enhertu, patients with HER2-low tumours may be eligible for HER2-directed therapy.
DESTINY-Breast04
DESTINY-Breast04 is a global, randomised, open-label, Phase III trial evaluating the efficacy and safety of Enhertu (5.4mg/kg) versus physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel) in patients with HR-positive or HR-negative, HER2-low unresectable and/or metastatic breast cancer previously treated with one or two prior lines of chemotherapy. Patients were randomised 2:1 to receive either Enhertu or chemotherapy.
The primary endpoint of DESTINY-Breast04 is PFS in patients with HR-positive disease based on blinded independent central review (BICR). Key secondary endpoints include PFS based on BICR in all randomised patients (HR-positive and HR-negative disease), OS in patients with HR-positive disease and OS in all randomised patients (HR-positive and HR-negative disease). Other secondary endpoints include PFS based on investigator assessment, objective response rate based on BICR and on investigator assessment, duration of response based on BICR and safety.
DESTINY-Breast04 enrolled 557 patients at multiple sites in Asia, Europe and North America. For more information about the trial, visit ClinicalTrials.gov.
Enhertu
Enhertu is a HER2-directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC technology, Enhertu is the lead ADC in the oncology portfolio of Daiichi Sankyo and the most advanced programme in AstraZeneca’s ADC scientific platform. Enhertu consists of a HER2 monoclonal antibody attached to a topoisomerase I inhibitor payload, an exatecan derivative, via a stable tetrapeptide-based cleavable linker.
Enhertu (5.4mg/kg) is approved in more than 30 countries for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received a (or one or more) prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy based on the results from the DESTINY-Breast03 trial.
Enhertu (5.4mg/kg) is approved in several countries for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens based on the results from the DESTINY-Breast01 trial.
Enhertu (5.4mg/kg) is approved in the US for the treatment of adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy based on the results from the DESTINY-Breast04 trial.
Enhertu (6.4mg/kg) is approved in several countries for the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who have received a prior trastuzumab-based regimen based on the results from the DESTINY-Gastric01 trial.
Enhertu development programme
A comprehensive development programme is underway globally, evaluating the efficacy and safety of Enhertu monotherapy across multiple HER2-targetable cancers, including breast, gastric, lung and colorectal cancers. Trials in combination with other anticancer treatments, such as immunotherapy, are also underway.
Regulatory applications for Enhertu in breast, gastric and non-small cell lung cancer are currently under review in several other countries based on the DESTINY-Breast01, DESTINY-Breast03, DESTINY-Breast04, DESTINY-Gastric01, DESTINY-Gastric02 and DESTINY-Lung01 trials, respectively.
Daiichi Sankyo collaboration
Daiichi Sankyo Company, Limited (TSE: 4568) [referred to as Daiichi Sankyo] and AstraZeneca entered into a global collaboration to jointly develop and commercialise Enhertu (a HER2-directed ADC) in March 2019, and datopotamab deruxtecan (DS-1062; a TROP2-directed ADC) in July 2020, except in Japan where Daiichi Sankyo maintains exclusive rights. Daiichi Sankyo is responsible for the manufacturing and supply of Enhertu and datopotamab deruxtecan.
AstraZeneca in breast cancer
Driven by a growing understanding of breast cancer biology, AstraZeneca is challenging and redefining the current clinical paradigm for how breast cancer is classified and treated to deliver even more treatments to patients in need – with the bold ambition to one day eliminate breast cancer as a cause of death.
AstraZeneca has a comprehensive portfolio of approved and promising compounds in development that leverage different mechanisms of action to address the biologically diverse breast cancer tumour environment.
AstraZeneca aims to continue to transform outcomes for HR-positive breast cancer with foundational medicines Faslodex (fulvestrant) and Zoladex (goserelin) and the next-generation oral selective oestrogen receptor degrader (SERD) and potential new medicine camizestrant.
PARP inhibitor Lynparza (olaparib) is a targeted treatment option that has been studied in HER2-negative early and metastatic breast cancer patients with an inherited BRCA mutation. AstraZeneca with MSD (Merck & Co., Inc. in the US and Canada) continue to research Lynparza in metastatic breast cancer patients with an inherited BRCA mutation and are exploring new opportunities to treat these patients earlier in their disease.
Building on the initial approvals of Enhertu, a HER2-directed ADC, in previously treated HER2-positive metastatic breast cancer, AstraZeneca and Daiichi Sankyo are exploring its potential in earlier lines of treatment and in new breast cancer settings.
To bring much needed treatment options to patients with triple-negative breast cancer, an aggressive form of breast cancer, AstraZeneca is testing immunotherapy Imfinzi (durvalumab) in combination with other oncology medicines, including Lynparza and Enhertu, evaluating the potential of AKT kinase inhibitor, capivasertib, in combination with chemotherapy, and collaborating with Daiichi Sankyo to explore the potential of TROP2-directed ADC, datopotamab deruxtecan.
AstraZeneca in oncology
AstraZeneca is leading a revolution in oncology with the ambition to provide cures for cancer in every form, following the science to understand cancer and all its complexities to discover, develop and deliver life-changing medicines to patients.
The Company’s focus is on some of the most challenging cancers. It is through persistent innovation that AstraZeneca has built one of the most diverse portfolios and pipelines in the industry, with the potential to catalyse changes in the practice of medicine and transform the patient experience.
AstraZeneca has the vision to redefine cancer care and, one day, eliminate cancer as a cause of death.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
- Modi S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022; 387:9-20.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V2.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 10.3322/caac.21660.
- Centers for Disease Control and Prevention. Available at: https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/. Accessed August 2022.
- American Cancer Society. Cancer Facts & Figures 2022. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html. Accessed August 2022.
- Iqbal N, et al. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int. 2014; 852748.
- Wolff A,et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018; 142(11): 1364-1382.
- Schalper K, et al. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas. Arch Pathol Lab Med. 2014; 138: 213-219.
- Schettini F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. npj Breast Cancer. 2021; 7:1; https://doi.org/10.1038/s41523-020-00208-2.
- Denkert C, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. 2021. Lancet Oncol; 22: 1151-61.
- Miglietta F, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021; 7: 137; 10.1038/s41523-021-00343-4.
- Matutino A, et al. Current Oncology. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. 2018; 25(S1):S131-S141.
- American Cancer Society. Breast Cancer Hormone Receptor Status. Available at: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-hormone-receptor-status.html. Accessed August 2022.
Dr. Modi has financial interests related to AstraZeneca and Daiichi Sankyo.
Marknadsnyheter
Teknisk analys på flera marknader med Anders Haglund
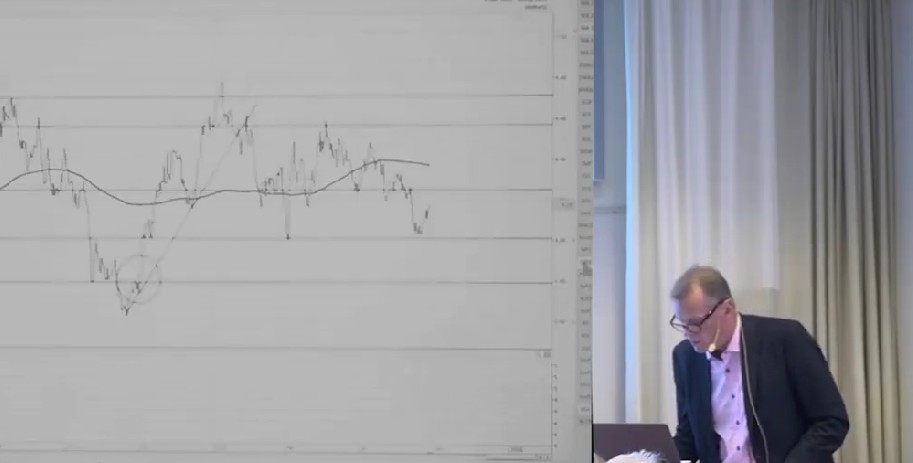
Anders Haglund går igenom den tekniska analysen på flera marknader samt även några olika enskilda aktier.
Marknadsnyheter
JRS chefsstrateg Torbjörn Söderberg om börsen framåt

JRS chefsstrateg Torbjörn Söderberg pratar med Jesper Norberg på EFN om börsens väg framåt. Man tar upp värderingar och makro, samt hur han själv väljer att agera.
Marknadsnyheter
Kreditkort skapar problem för USAs konsumenter – CNBC granskar

CNBC tittar närmare på hur kreditkort skapar problem för konsumenterna i USA som får betala räntor på upp till 36 %, och ovanpå det kommer nya avgifter. När det skapar så här stora problem blir det ett problem för ekonomin som helhet, det är inte bara ett individuellt problem.
CNBC granskar kreditkort och problemen de skapar
Vi skrev nyligen om rekordhög belåning hos investerare i USA. Det är samma sak här, när det är så många individer som är så hårt belånade blir det ett problem för hela aktiemarknaden.
-
Analys från DailyFX10 år ago
EUR/USD Flirts with Monthly Close Under 30 Year Trendline
-
Marknadsnyheter5 år ago
BrainCool AB (publ): erhåller bidrag (grant) om 0,9 MSEK från Vinnova för bolagets projekt inom behandling av covid-19 patienter med hög feber
-

 Marknadsnyheter3 år ago
Marknadsnyheter3 år agoUpptäck de bästa verktygen för att analysera Bitcoin!
-
Analys från DailyFX12 år ago
Japanese Yen Breakout or Fakeout? ZAR/JPY May Provide the Answer
-

 Marknadsnyheter2 år ago
Marknadsnyheter2 år agoDärför föredrar svenska spelare att spela via mobiltelefonen
-
Analys från DailyFX12 år ago
Price & Time: Key Levels to Watch in the Aftermath of NFP
-
Analys från DailyFX8 år ago
Gold Prices Falter at Resistance: Is the Bullish Run Finished?
-
 Nyheter7 år ago
Nyheter7 år agoTeknisk analys med Martin Hallström och Nils Brobacke

